Saturday, August 30, 2014
Friday, August 29, 2014
What is DTSI Technology?
What is DTSI Technology?
Ans. DTSI stands for Digital Twin Spark Plug Ignition. The vehicles with DTSI Technology use 2 spark plugs which are controlled
by digital circuit. It results in efficient combustion of air fuel mixture.
• Digital - Since the spark generation will be initiated by a microchip.
• Twin - Since two spark plugs will be used.
• Spark ignition - Since the ignition will be done via a spark
Ans. DTSI stands for Digital Twin Spark Plug Ignition. The vehicles with DTSI Technology use 2 spark plugs which are controlled
by digital circuit. It results in efficient combustion of air fuel mixture.
• Digital - Since the spark generation will be initiated by a microchip.
• Twin - Since two spark plugs will be used.
• Spark ignition - Since the ignition will be done via a spark
Thursday, August 28, 2014
recent education style in my locality
"some say that they make good discipline students".These people make students narrow minded,well dressed,spiritual and not having the boldness to say what is wrong and how to select a good candidate even in election,mislead by poor lectures who had their poor knowledge and making their satisfactionary life ,boasting that they had suceded from where they came from.They teach us how much stress a rod can withstand but they dont how much stress that a student can withstand ,what type of stresses are induced in their brain....they gurantee a job where i have to do the same like them and saying
"yes yes yes " to my higher officials and iam going to continue ttheir dreams and not my dreams" ....all they need is say "yes" and respect them
"yes yes yes " to my higher officials and iam going to continue ttheir dreams and not my dreams" ....all they need is say "yes" and respect them
Wednesday, August 27, 2014
WHAT IS MODULUS OF RIGIDITY??????????????????
We knew from Hook's law- "stress is proportional to strain." So, stress = k * strain [here, k is a constant] or, stress/strain= k Now, If the stress and strain occurs due to axial force then k is known as modulus of elasticity and it is denoted by E. if the stress and strain occurs due to shear force then k is known as modulus of rigidity and it is denoted by G.
Tuesday, August 26, 2014
Sunday, August 24, 2014
Friday, August 22, 2014
Thursday, August 21, 2014
Saturday, August 16, 2014
The first moment of area equals the summation of area times distance to an axis [Σ(a x r)]. It is a measure of the distribution of the area of a shape in relationship to an axis.
First moment of area is commonly used in engineering applications to determine the centroid of an object or the statical moment of area.
b) The second moment of area, also known as the area moment of inertia or second moment of inertia, is a property of a shape that is used to predict its resistance to bending and deflection which are directly proportional.
second moment of area= Ar^2
c) Mass moment of inertia or the angular mass, is the rotational analog of mass. That is, it is the inertia of a rigid rotating body with respect to its rotation.
Here, its again the very old discussion. The above part is second moment of area. Here, its of mass.
Mass Moment of inetia = mr^2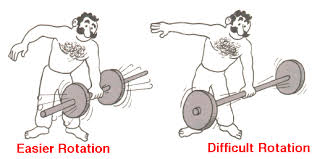 -PROFESSOR PRADEEP KARNA
-PROFESSOR PRADEEP KARNA
First moment of area is commonly used in engineering applications to determine the centroid of an object or the statical moment of area.
b) The second moment of area, also known as the area moment of inertia or second moment of inertia, is a property of a shape that is used to predict its resistance to bending and deflection which are directly proportional.
second moment of area= Ar^2
c) Mass moment of inertia or the angular mass, is the rotational analog of mass. That is, it is the inertia of a rigid rotating body with respect to its rotation.
Here, its again the very old discussion. The above part is second moment of area. Here, its of mass.
Mass Moment of inetia = mr^2
MOMENT OF INERTIA
 what does this diagram say to you????
what does this diagram say to you????ans: by changing the orientation of the beam ,bending of the beam can be avoided.Excess bending causes failure in order to use a number value for describing the bending of a particular geometry second moment of inertia is used.It is denoted by "I"
stress????
SIMPLE STRESS
STRESS is the intensity of force inside a solid. The basic unit of stress is the Pascal (Pa) which is Newton per square metre. In engineering it is more convenient to measured as the force (N) per square mm. This gives the common engineering unit of stress, MPa.
Tensile, Compressive and Shear stress
There are 3 types of stress in the world;
- Tensile = pulling apart
- Compressive = squashing together
- Shear = sliding apart
Any of these 3 types of stress are calculated the same way, with the same units - it the area that is different. Always think of what area must be broken when the component fails (the broken area).
What is a Stress?
STRESS is the intensity of force inside a solid.
It has the same units as Pressure (Pa, kPa, MPa, etc), so you could think of stress as pressure in a solid. The difference is, pressure acts equally in every direction, but stress has a certain direction.
Stress = Force/Area
The base unit for pressure and stress is the Pascal (Pa), but this is way too small for engineering use - except perhaps when measuring the pressure of air conditioning ducts or something. Certainly nothing compared to the stress required to break steel. In most engineering situations, the strength of a material is measured in MPa (MegaPascals)
Stress (MPa) = Force (N) / Area (mm2)
| DEFINE | Formula | Units | Diagram |
| Axial Stress (Tension or Compression) | Stress = Force / Area | MPa | |
| Axial Strain (Tension or Compression) | Strain = extension / original Length | - | |
| Shear Stress | Stress = Force / Area | MPa | |
| Modulus of Elasticity (Young's Mod) | E = Stress / Strain | GPa | Slope of Stress:Strain diagram |
| Modulus of Rigidity (Shear Mod.) =~ 0.4E | G = S. Stress / S. Strain | GPa | Slope of S.Stress:S.Strain diagram |
| Shear Strain | Strain = movement / original Depth | - | |
| Shear in Detail: Shear Strain is usually small enough to ignore the changes in L with angle. Angle is in radians. Area is the zone that would slide apart assuming it broke in shear. | |||
Friday, August 15, 2014
Wednesday, August 13, 2014
Sunday, August 10, 2014
what is quassi static process?????
Quasi means almost or near to. Quasi–Static process means very nearly static process. Let us consider a system of gas contained in cylinder. The gas held by a moving piston. A weight W is placed over the piston. Due to the weight , the gas in cylinder is compressed. After the gas reaches equilibrium, the properties of gas are denoted by p1, v1, t1. The weight placed over the piston is balanced by upward force exerted by the gas. If the weight is removed, then there will be unbalanced force between the system and the surroundings. The gas under pressure will expand and push the piston upward till it touches the stops. The properties at this state after reaching equilibrium are p2,v2,t2. But the intermediate states passed through by the system are non-equilibrium states which cannot be described by thermodynamic coordinates. In this case we only have initial and final states and do not have a path connecting them.
Suppose, the weight is made of large numbers of small weights. And one by one each of these small weights are removed and allowed the system to reach an equilibrium state. Then we have intermediate equilibrium states and the path described by these sates will not deviate much from the thermodynamic equilibrium state. Such a process, which is the locus of all the intermediate points passed by the system is known as Quasi-static process. It means, this process is almost near to the thermodynamically equilibrium process. Infinite slowness is the characteristic feature of quasi-static process.
Thursday, August 7, 2014
exergy anergy entropy
Ask a number of people what they assume energy is. You will have as much different answers as the number of people. So, what is energy? Well, -energy is the capacity to do work- and this is available in a lot of different forms, p.e. electrical, chemical, potential, etc. However not all energy available can be transformed into work. Only a part of it can. Why is this so? Consider therefore a heat source of some kind. This heat can be used to do work and will cool down in doing so. However it can only do this until the temperature is equal to the surrounding, not to a lower value because you would need work in getting the temperature back up. The work obtained from the heat source until the temperature of the surroundings is the amount of exergy. This is a smaller value than the energy what was available in the beginning and the difference is the anergy, the part which is unusable. So in short: energy=exergy+anergy, with exergy the usefull part and anergy the unusable part.
In the process of transforming one kind of energy into the other we have an exergy loss. This means that during the process some exergy is lost and is transformed into anergy. Assume as a simple example that you have 100J energy of which is 80J exergy and 20J anergy in the beginning. After transforming this energy to another form of energy you still have 100J at the end, but only 40J of exergy and 60J of anergy. This means that 40J of exergy is transformed into anergy. A part of the usefull energy is tranformed into unusable anergy. The fact that the energy is constant is nothing more than the first law of thermodynamics. The loss in exergy is a manifestation of the second law. The fact that there is some amount of anergy in p.e. a heat source is the reason why we can't use the energy in the sea, it is all anergy, at least to some extent. To what temperature must we cool it down, it has allready the temperature of the surrounding? A mistake often made. There is energy which is (almost) completely transformable into work meaning that is has no anergy, this is electrical energy, work itself is also exergy.
Exergy and anergy are related to entropy, the amount of entropy rise is related to the loss of exergy. That's one reason why understanding entropy is so important. Be carefull in using this, the entropy can drop in a part of a system, but must always rise for the complete system, often the surroundings entropy rises more than the drop of the system's part.
In the process of transforming one kind of energy into the other we have an exergy loss. This means that during the process some exergy is lost and is transformed into anergy. Assume as a simple example that you have 100J energy of which is 80J exergy and 20J anergy in the beginning. After transforming this energy to another form of energy you still have 100J at the end, but only 40J of exergy and 60J of anergy. This means that 40J of exergy is transformed into anergy. A part of the usefull energy is tranformed into unusable anergy. The fact that the energy is constant is nothing more than the first law of thermodynamics. The loss in exergy is a manifestation of the second law. The fact that there is some amount of anergy in p.e. a heat source is the reason why we can't use the energy in the sea, it is all anergy, at least to some extent. To what temperature must we cool it down, it has allready the temperature of the surrounding? A mistake often made. There is energy which is (almost) completely transformable into work meaning that is has no anergy, this is electrical energy, work itself is also exergy.
Exergy and anergy are related to entropy, the amount of entropy rise is related to the loss of exergy. That's one reason why understanding entropy is so important. Be carefull in using this, the entropy can drop in a part of a system, but must always rise for the complete system, often the surroundings entropy rises more than the drop of the system's part.
Wednesday, August 6, 2014
Saturday, August 2, 2014
first law and second law of thermodynamics
The Conservation of Energy and the First Law of Thermodynamics
Our changeable friend energy, with its multiple "personalities", is sometimes hard to keep track of and even harder to define. But it does always behave in predictable ways. This means that if you know the rules you, like engineers and scientists, can often predict what will happen and what won't happen.
Smart scientists and engineers, through clever experiments and even cleverer thinking, figured them out in days gone by. It took some open-minded creative thinking. It was not obvious to anyone back then. And much of what was obvious back then about energy was wrong.
The First Law:
During all this moving and transforming the total amount of energy never changes.
That's it! I've done it!
That's the first law of thermodynamics! Ta da!
Energy changes form and moves from place to place but the total amount doesn't change.
Simple, eh? Other ways to say it:
Other ways to say it:
It's conservation of energy. Energy is conserved during any and every process or transformation or "happening".
Energy doesn't pop into existence from nowhere.
Energy doesn't pop out of existence into nowhere.
Energy is neither created nor destroyed (the old fuddy duddy way of saying it).
"Energy in" equals "energy stored" plus "energy out"
(if no energy is stored, then "Energy in" equals "Energy out").
You may have already heard that the 1st Law says energy is "always conserved". So why are we always saying we need to conserve more energy if it is "always conserved" already? Darn old english language, same word but different meanings. When we say energy is always conserved we mean the total amount of energy in any process or reaction never changes. When we tell ourselves to conserve energy we mean to use less of it by doing things like driving more fuel efficient cars, or insulating our houses better.
 But hold on, if energy never goes away, if the total amount always stays the same, why do we have to worry about not wasting it? Can't we just keep reusing it? We don't really "use" energy, we convert it, and it never "get's used up".
But hold on, if energy never goes away, if the total amount always stays the same, why do we have to worry about not wasting it? Can't we just keep reusing it? We don't really "use" energy, we convert it, and it never "get's used up".
It's not a stupid question. It's a very good question. Unfortunately, the First Law of Thermo doesn't really answer it. The popular first law just says the total amount of energy doesn't change.
Scientists and Engineers had to discover a second law of thermodynamics to answer questions like those above. This section is only on the first law but you can jump to the Second Law page now for a very good explanation of the gist of that law, or continue on to the fascinating next page for some good examples to further clarify the First Law. I hope you will read all the pages, for the sake of my fragile ego.
 On page two of this section we'll give some examples of the results of the conservation of energy. Click on the Next Page link below.
On page two of this section we'll give some examples of the results of the conservation of energy. Click on the Next Page link below.
Our changeable friend energy, with its multiple "personalities", is sometimes hard to keep track of and even harder to define. But it does always behave in predictable ways. This means that if you know the rules you, like engineers and scientists, can often predict what will happen and what won't happen.
Smart scientists and engineers, through clever experiments and even cleverer thinking, figured them out in days gone by. It took some open-minded creative thinking. It was not obvious to anyone back then. And much of what was obvious back then about energy was wrong.
This section is on the First Law of Thermodynamics, or the Conservation of Energy as it is often called. To start, let's remind ourselves of a few things. A Few things to remember: Energy can be stored. Energy can move from one bunch or piece of matter to another bunch or piece of matter. Energy can be transformed from one type of energy to another type of energy. |  | |||||
| A wind turbine converts some, not all, of the kinetic energyin the wind into mechanical and electrical energy. The First Law of Thermodynamics says the sum of the energy put into the wind turbine plus the remaining energy in the air after it passes through the turbine, must exactly equal the energy in the wind before it entered the turbine. | ||||||
During all this moving and transforming the total amount of energy never changes.
That's it! I've done it!
That's the first law of thermodynamics! Ta da!
Energy changes form and moves from place to place but the total amount doesn't change.
Simple, eh?
 Other ways to say it:
Other ways to say it:It's conservation of energy. Energy is conserved during any and every process or transformation or "happening".
Energy doesn't pop into existence from nowhere.
Energy doesn't pop out of existence into nowhere.
Energy is neither created nor destroyed (the old fuddy duddy way of saying it).
"Energy in" equals "energy stored" plus "energy out"
(if no energy is stored, then "Energy in" equals "Energy out").
You may have already heard that the 1st Law says energy is "always conserved". So why are we always saying we need to conserve more energy if it is "always conserved" already? Darn old english language, same word but different meanings. When we say energy is always conserved we mean the total amount of energy in any process or reaction never changes. When we tell ourselves to conserve energy we mean to use less of it by doing things like driving more fuel efficient cars, or insulating our houses better.
 But hold on, if energy never goes away, if the total amount always stays the same, why do we have to worry about not wasting it? Can't we just keep reusing it? We don't really "use" energy, we convert it, and it never "get's used up".
But hold on, if energy never goes away, if the total amount always stays the same, why do we have to worry about not wasting it? Can't we just keep reusing it? We don't really "use" energy, we convert it, and it never "get's used up".It's not a stupid question. It's a very good question. Unfortunately, the First Law of Thermo doesn't really answer it. The popular first law just says the total amount of energy doesn't change.
Scientists and Engineers had to discover a second law of thermodynamics to answer questions like those above. This section is only on the first law but you can jump to the Second Law page now for a very good explanation of the gist of that law, or continue on to the fascinating next page for some good examples to further clarify the First Law. I hope you will read all the pages, for the sake of my fragile ego.
 On page two of this section we'll give some examples of the results of the conservation of energy. Click on the Next Page link below.
On page two of this section we'll give some examples of the results of the conservation of energy. Click on the Next Page link below. | Energy is Conserved but Not Its Usefulness As we said above, the first law of thermodynamics says the total amount of energy never changes, even after many changes. It doesn't answer questions like "why can't we re-use the energy over and over again?" or "where do my son's guitar picks go?" Alas, the second law does answer the questions about re-using energy (not the one's about missing guitar picks and socks though, those mysteries are still beyond the realm of science). The Second Law is not as easy to say as the first law. It can't be described in one or two sentences. We can't cover it all here, but we can give a brief answer to the questions above. The second law deals with the availability or useability of energy. The unhappy truth is that as energy gets used or transformed in machines or living animals it gets converted into a form that is less useful than before. Something in the energy does get "used up" or even "destroyed". That something is what you might call "usefulness" or as it is often called in thermodynamics, "availability". The energy becomes less available for us to use. In engines, like those in cars, trucks, locomotives, and airplanes, the energy in the fuel is highly concentrated, meaning there is a lot of energy in a fairly small light package. Each gallon of gasoline (petrol for those of you in other lands) or diesel fuel or jet fuel contains a lot of concentrated easy to use energy. Once that fuel is burned in the engines we are all so dependant on, it gets converted into highly concentrated thermal energy (high temperature) and mechanical energy. The mechanical energy is used to do useful work for us like moving people or freight from one place to another. But eventually all of the fuel's concentrated useful energy get's changed into "low grade" thermal energy. Most is converted immediately into "heat" and goes out of the engine in the cooling water or the engine's hot exhaust gas. The useful mechanical energy gets turned into heat through friction of moving parts or by pushing air molecules out of the way. The molecules that get pushed out of the way absorb the energy of the car or train as increased kinetic energy of the air molecules. All of the fuel's formerly concentrated "high grade" energy, becomes what we engineer's call "low grade" energy or "waste" heat. We call it that because it is no longer useful to us. It's there, but it's sort of messy and all spread out now, to "gather it up" would take more energy than it's worth. To reuse that low grade energy is simply impossible or would be too expensive and impractical to contemplate. So there it goes, all absorbed into the air and the objects around us as low temperature low grade energy which will finally radiate into outer space to help warm up the universe a tiny little bit. We have to replenish it by burning more fuel. If the fuel is what we call fossil fuel it will eventually all be gone, along with all of its cheap easy to use energy. Since we haven't yet figured out how to use other forms of energy or alternative fuels as well yet, and those alternatives are likely to be more expensive, most of us think it should not be wasted. That's why we talk about the need to conserve energy. The first law tells us that energy is forever and the total amount stays the same. The second law tells us that "high grade" useful energy always gets turned into "low grade" less useful energy that is difficult or impossible to re-use. The same is true of the energy conversions in living organisms. The food you eat eventually gets converted into heat (thermal energy) and mechanical work. The heat is useful for a little while to help keep your body temperature constant. The mechanical work is used to make more cells, to breathe, think, run, grow, etc. But in the end, just like with our human made machines, all of the concentrated high grade food energy gets converted into low grade energy we can't reuse. All of it eventually ends up as thermal energy (often called heat) spread out into our surroundings. This also ends up getting radiated into space. And, as you know if you've read the photosynthesis pages, we would run out of useful energy for life very quickly if it didn't get replenished every day by a fresh dose of concentrated energy from the sun. Back to Where You Were * * source: http://www.ftexploring.com/energy/first-law.html |
Subscribe to:
Comments (Atom)

